- Product Overview
- Claims Reviewed
- Supplement Facts
- Research Transparency™
- How to Use Certification
Mdrive™ Classic is a dietary supplement for men ages 40+ that supports normal healthy testosterone levels and improves cardiorespiratory functions. It also includes ashwagandha, called an adaptogen, an herbal extract that helps normalize body imbalances and reduce stress.
Level 2 – Claims
- Energy, stamina, strength, drive
- Reduces stress
- Maintains healthy cortisol levels
- Testosterone support
- Beta-Sitosterols to support prostate health
- Maintains lean muscle mass
- Improves cardio-respiratory function
- Supports healthy libido

STRONGSCIENCE Product Review MDrive™ Classic Product
8. REFERENCES Mdrive Classic
Vitamin D3 (cholecalciferol)
1. Natural Medicines Comprehensive Database
http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?cs=&s=ND&pt=9&Product=Ashwagandha&btnSearch.x=7&btnSearch.y=10, last updated on 10/31/2017.
2. Vitamin D dosing: an update. Pharmacist’s Letter/Prescriber’s Letter 2010;26(7):260707.
3. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-6. View abstract.
4. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992;327:1637-42. View abstract.
5. Minne HW, Pfeifer M, Begerow B, et al. Vitamin D and calcium supplementation reduces falls in elderly women via improvement of body sway and normalization of blood pressure: a prospective, randomized, and double-blind study. Abstracts World Congress on Osteoporosis 2000.
6. Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int 2002;13:257-64.. View abstract.
7. Meyer H, Smedshaug GB, Kvaavik E, et al. Can vitamin D supplementation reduce the risk fracture in the elderly? A randomized controlled trial. J Bone Miner Res 2002;17:709-15. View abstract.
8. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785-95. View abstract.
9. National Osteoporosis Foundation. Physician’s Guide to Prevention and Treatment of Osteoporosis. Universal Recommendations for All Patients. Available at: http://www.nof.org/physguide/univeral_recommendations.htm#adequate. (Accessed 14 May 2005).
10. Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res 2004;19:370-8. View abstract.
11. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257-64. View abstract
12. Boonen S, Body JJ, Boutsen Y, et al. Evidence-based guidelines for the treatment of postmenopausal osteoporosis: a consensus document of the Belgian Bone Club. Osteoporos Int 2005;16:239-54. View abstract.
13. Papadimitropoulos E, Wells G, Shea B, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 2002;23:560-9. View abstract.
14. Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713-6. View abstract.
15. Jackson RD, LaCroix AZ, Gass M. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669-83. View abstract.
16. Boonen S, Lips P, Bouillon R, et al. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab 2007;92:1415-23. View abstract.
17. New 2010 Vitamin D Recommendations. Osteoporosis Canada, July 2010. Available at: http://www.osteoporosis.ca/index.php/ci_id/5536/la_id/1.htm.
18. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press, 1999. Available at: http://books.nap.edu/books/0309063507/html/index.html.
19. Diamond TH, Ho KW, Rohl PG, Meerkin M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Med J Aust 2005;183:10-2. View abstract.
20. Cava RC, Javier AN. Vitamin D deficiency [editorial]. N Engl J Med 2007;357:1981. View abstract.
21. Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D Deficiency – Is There Really a Pandemic? N Engl J Med. 2016 ;375(19):1817-1820. View abstract
22. Balion, C., Griffith, L. E., Strifler, L., Henderson, M., Patterson, C., Heckman, G., Llewellyn, D. J., and Raina, P. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology 9-25-2012;79(13):1397-1405. View abstract.
23. Kenny AM, Biskup B, Robbins B, et al. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc 2003;51:1762-7. View abstract.
24. Stockton, K. A., Mengersen, K., Paratz, J. D., Kandiah, D., and Bennell, K. L. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos.Int 2011;22(3):859-871. View abstract.
25. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014 Nov;99(11):4336-45. View abstract.
26. Pfeifer, M., Begerow, B., Minne, H. W., Suppan, K., Fahrleitner-Pammer, A., and Dobnig, H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos.Int 2009;20(2):315-322. View abstract.
27. Zhu, K., Austin, N., Devine, A., Bruce, D., and Prince, R. L. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr.Soc. 2010;58(11):2063-2068. View abstract.
28. Dhesi, J. K., Jackson, S. H., Bearne, L. M., Moniz, C., Hurley, M. V., Swift, C. G., and Allain, T. J. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing 2004;33(6):589-595. View abstract.
29. Nimptsch K., Platz E.A., Willett W. C., Giovannucci E. “Association Between Plasma 25-OH Vitamin D and Testosterone Levels in Men.” Clinical Endocrinology (2012) 77: pp. 106-112
30. Pilz S., Frisch S, Koertke H., Kuhn J., Dreier J., Obermayer-Pietsch B., Wehr E., Zittermann A., “Effects of Vitamin D Supplementation on Testosterone Levels in Men.” Hormone Metabolism Research (2010) 43: pp. 223-225
31. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press, 1999. Available at: http://books.nap.edu/books/0309063507/html/index.html.
32. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 2004;79:362-71.. View abstract.
33. Shearer MJ. The roles of vitamins D and K in bone health and osteoporosis prevention. Proc Nutr Sci 1997;56:915-37. View abstract
34. Need AG, Horowitz M, Morris HA, Nordin BEC. Vitamin D status: effects on parathyroid hormone and 1,25-dihydroxyvitamin D in postmenopausal women. Am J Clin Nutr 2000;71:1577-81. View abstract
35. Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int 2002;13:187-94. . View abstract
36. Prabhala A, Garg R, Dandona P. Severe myopathy associated with vitamin D deficiency in western New York. Arch Intern Med 2000; 160:1199–203. . View abstract.
37. Pfeifer M, Begerow B, Minne HW, et al. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res 2000;15:1113-8. View abstract.
38. Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res 2002;17:891-7. . View abstract.
39. Baker K, Zhang YQ, Goggins J, et al. Hypovitaminosis D and its association with muscle strength, pain, and physical function in knee osteoarthritis (OA). American College of Rheumatology Meeting; San Antonio, Texas, October 16-21, 2004. Abstract 1755.
40. Merlino LA, Curtis J, Mikuls TR, et al. Vitamin D intake is inversely associated with rheumatoid arthritis. Arthritis Rheum 2004;50:72-7. View abstract.
41. Dietary reference intakes for calcium and vitamin D. Institute of Medicine, November 30, 2010. Available at: http://www.iom.edu/~/media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20Calcium%202010%20Report%20Brief.pdf.
Vitamin B12
42. McEvoy GK, ed. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, 1998.
43. Paltiel O, Falutz J, Veilleux M, et al. Clinical correlates of subnormal vitamin B12 levels in patients infected with the human immunodeficiency virus. Am J Hematol 1995;49:318-22. View abstract.
44. Carlsen SM, Folling I, Grill V, et al. Metformin increases total homocysteine levels in non-diabetic male patients with coronary heart disease. Scand J Clin Lab Invest 1997;57:521-7. View abstract.
45. Kanai T, Takagi T, Masuhiro K, et al. Serum vitamin K level and bone mineral density in post-menopausal women. Int J Gynaecol Obstet 1997;56:25-30. View abstract.
46. Singh RB, Niaz MA, Sharma JP, et al. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival-4. Cardiovasc Drugs Ther 1997;11:485-91. View abstract.
47. Yamadera W, Sasaki M, Itoh H, et al. Clinical features of circadian rhythm sleep disorders in outpatients. Psychiatry Clin Neurosci 1998;52:311-6. View abstract.
48. Okawa M, Uchiyama M, Ozaki S, et al. Circadian rhythm sleep disorders in adolescents: clinical trials of combined treatments based on chronobiology. Psychiatry Clin Neurosci 1998;52:483-90. View abstract.
49. Okawa M, Takahashi K, Egashira K, et al. Vitamin B12 treatment for delayed sleep phase syndrome: a multi-center double-blind study. Psychiatry Clin Neurosci 1997;51:275-9. View abstract.
50. Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake rhythm disorders: therapeutic effects of vitamin B12, bright light therapy, chronotherapy and hypnotics. Psychiatry Clin Neurosci 1996;50:203-9. View abstract.
51. Ohta T, Ando K, Iwata T, et al. Treatment of persistent sleep-wake schedule disorders in adolescents with methylcobalamin (vitamin B12). Sleep 1991;14:414-8. View abstract.
52. Mayer G, Kroger M, Meier-Ewert K. Effects of vitamin B12 on performance and circadian rhythm in normal subjects. Neuropsychopharmacology 1996;15:456-64. View abstract.
53. Houston DK, Johnson MA, Nozza RJ, et al. Age-related hearing loss, vitamin B-12, and folate in elderly women. Am J Clin Nutr 1999;69:564-71. View abstract.
54. Kuzminski AM, Del Giacco EJ, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood 1998;92:1191-1198. View abstract.
55. Andres E, Kurtz JE, Perrin AE, et al. Oral cobalamin therapy for the treatment of patients with food-cobalamin malabsorption. Am J Med 2001;111:126-9. View abstract.
56. Lederle FA. Oral cobalamin for pernicious anemia. Medicine’s best kept secret? JAMA 1991;265:94-5. View abstract.
57. Elia M. Oral or parenteral therapy for B12 deficiency. Lancet 1998;352:1721-2. View abstract.
58. Andres E, Goichot B, Schlienger JL. Food cobalamin malabsorption: a usual cause of vitamin B12 deficiency. Arch Intern Med 2000;160:2061-2. View abstract.
59. Verhaeverbeke I, Mets T, Mulkens K, Vandewoude M. Normalization of low vitamin B12 serum levels in older people by oral treatment. J Am Geriatr S
60. Allen LH, Casterline J. Vitamin B-12 deficiency in elderly individuals: diagnosis and requirements. Am J Clin Nutr 1994;60:12-14. View abstract.
61. Hathcock JN, Troendle GJ. Oral cobalamin for treatment of pernicious anemia? JAMA 1991;265:96-97. View abstract.
62. Line DH, Seitanidis B, Morgan JO, Hoffbrand AV. The effects of chemotherapy on iron, folate, and vitamin B12 metabolism in tuberculosis. Q J Med 1971;40:331-40. View abstract.
63. Rajan, S., Wallace, J. I., Brodkin, K. I., Beresford, S. A., Allen, R. H., and Stabler, S. P. Response of elevated methylmalonic acid to three dose levels of oral cobalamin in older adults. J Am Geriatr.Soc 2002;50(11):1789-1795. View abstract.
64. Kaltenbach, G., Noblet-Dick, M., Andres, E., Barnier-Figue, G., Noel, E., Vogel, T., Perrin, A. E., Martin-Hunyadi, C., Berthel, M., and Kuntzmann, F. [Early response to oral cobalamin therapy in older patients with vitamin B12 deficiency]. Ann.Med.Interne (Paris) 2003;154(2):91-95. View abstract.
65. Bolaman, Z., Kadikoylu, G., Yukselen, V., Yavasoglu, I., Barutca, S., and Senturk, T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clin Ther 2003;25(12):3124-3134. View abstract.
66. Kaltenbach, G., Noblet-Dick, M., Andres, E., Barnier-Figue, G., Noel, E., and Vogel, T. Reponse precoce au traitement oral par vitamine B12 chez des sujets ages hypovitaminiques. Annales de Medecine Interne (Paris) 2003;154:91-95.
67. E., Kaltenbach, G., Noblet-Dick, M., Noel, E., Vinzio, S., Perrin, A. E., Berthel, M., and Blickle, J. F. Hematological response to short-term oral cyanocobalamin therapy for the treatment of cobalamin deficiencies in elderly patients. J Nutr Health Aging 2006;10(1):3-6. View abstract.
68. Eussen SJ, de Groot LC, Clarke R, et al. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: A dose-finding trial. Arch Intern Med 2005;165:1167-72. View abstract.
69. McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med 2006;354:2764-72. View abstract.
70. van der Zwaluw NL, Dhonukshe-Rutten RA, van Wijngaarden JP, et al. Results of a 2-year vitamin B treatment on cognitive performance: secondary data from an RCT. Neurology 2014;83(23):2158-66. View abstract.
71. Kwok T, Tang C, Woo J, et al. Randomized trial of the effect of supplementation on the cognitive function of older people with subnormal cobalamin levels. Int J Geriatr Psychiatry 1998;13:611-6.
72. McEvoy GK, ed. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, 1998.
73. Stehouwer CD. Clinical relevance of hyperhomocysteinaemia in atherothrombotic disease. Drugs and Aging 2000;16:251-60.. View abstract.
74. Yamadera W, Sasaki M, Itoh H, et al. Clinical features of circadian rhythm sleep disorders in outpatients. Psychiatry Clin Neurosci 1998;52:311-6. View abstract.
75. Okawa M, Uchiyama M, Ozaki S, et al. Circadian rhythm sleep disorders in adolescents: clinical trials of combined treatments based on chronobiology. Psychiatry Clin Neurosci 1998;52:483-90. View abstract.
76. Okawa M, Takahashi K, Egashira K, et al. Vitamin B12 treatment for delayed sleep phase syndrome: a multi-center double-blind study. Psychiatry Clin Neurosci 1997;51:275-9. View abstract.
77. Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake rhythm disorders: therapeutic effects of vitamin B12, bright light therapy, chronotherapy and hypnotics. Psychiatry Clin Neurosci 1996;50:203-9. View abstract.
78. Ohta T, Ando K, Iwata T, et al. Treatment of persistent sleep-wake schedule disorders in adolescents with methylcobalamin (vitamin B12). Sleep 1991;14:414-8. View abstract.
79. Hathcock JN, Troendle GJ. Oral cobalamin for treatment of pernicious anemia? JAMA 1991;265:96-97. View abstract.
80. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (2000). Washington, DC: National Academy Press, 2000. Available at: http://books.nap.edu/books/0309065542/html/.
81. Benito-Leon J, Porta-Etessam J. Shaky-leg syndrome and vitamin B12 deficiency. N Engl J Med 2000;342:981. View abstract.
82. Booth GL, Wang EE. Preventive health care, 2000 update: screening and management of hyperhomocysteinemia for the prevention of coronary artery disease events. The Canadian Task Force on Preventive Health Care. CMAJ 2000;163:21-9. View abstract.
83. Tremblay R, Bonnardeaux A, Geadah D, et al. Hyperhomocystinemia in hemodialysis patients: effects of 12-month supplementation with hydrosoluble vitamins. Kidney Int 2000;58:851-8. View abstract.
84. Manns B, Hyndman E, Burgess E, et al. Oral vitamin B(12) and high-dose folic acid in hemodialysis patients with hyper-homocyst(e)inemia. Kidney Int 2001;59:1103-9. View abstract.
85. Beaulieu AJ, Gohh RY, Han H, et al. Enhanced reduction of fasting total homocysteine levels with supraphysiological versus standard multivitamin dose folic acid supplementation in renal transplant recipients. Arterioscler Thromb Vasc Biol 1999;19:2918-21. View abstract.
86. Deutch B, Jorgensen EB, Hansen JC. n-3 PUFA from fish or seal oil reduce atherogenic risk indicators in Danish women. Nutr Res 2000;20:1065-77.
87. Bostom A, Shemin D, Gohh R, et al. Treatment of mild hyperhomocysteinemia in renal transplant recipients versus hemodialysis patients. Transplantation 2000;69:2128-31. View abstract
88. Dierkes J, Domrose U, Bosselmann P, et al. Homocysteine lowering effect of different multivitamin preparations in patients with end-stage renal disease. J Renal Nutr 2001;11:67-72. View abstract.
89. Bostom AG, Shemin D, Gohh RY, et al. Treatment of hyperhomocysteinemia in hemodialysis patients and renal transplant recipients. Kidney Int 2001;59:s246-s252. View abstract.
90. Seal EC, Metz J, Flicker L, Melny J. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc 2002;50:146-51. View abstract.
91. McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med 2006;354:2764-72. View abstract.
92. Stucker M, Pieck C, Stoerb C, et al. Topical vitamin B12 – a new therapeutic approach in atopic dermatitis – evaluation of efficacy and tolerability in a randomized placebo-controlled multicentre clinical trial. Br J Dematol 2004;150:977-83. View abstract
93. Uhl, W., Nolting, A., Golor, G., Rost, K. L., and Kovar, A. Safety of hydroxocobalamin in healthy volunteers in a randomized, placebo-controlled study. Clin Toxicol (Phila) 2006;44 Suppl 1:17-28. View abstract.
94. Rocco A, Compare D, Coccoli P, et al. Vitamin B12 supplementation improves rates of sustained vital response in patients chronically infected with hepatitis C virus.
Vitamin B6
95. McEvoy GK, ed. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, 1998.
96. Fishman SM, Christian P, West KP. The role of vitamins in the prevention and control of anaemia. Public Health Nutr 2000;3:125-50.. View abstract.
97. Mayer EL, Jacobsen DW, Robinson K. Homocysteine and coronary atherosclerosis. J Am Coll Cardiol 1996;27:517-27. View abstract
98. Selhub J, Jacques PF, Bostom AG, et al. Relationship between plasma homocysteine and vitamin status in the Framingham study population. Impact of folic acid fortification. Publ Health Rev 2000;28:117-45. View abstract.
99. Sunder-Plassmann G, Winkelmayer WC, Fodinger M. Therapeutic potential of total homocysteine-lowering drugs on cardiovascular disease. Expert Opin Investig Drugs 2000;9:2637-51. View abstract.
100. Woodside JV, Yarnell JW, McMaster D, et al. Effect of B-group vitamins and antioxidant vitamins on hyperhomocysteinemia: a double-blind, randomized, factorial-design, controlled trial. Am J Clin Nutr 1998;67:858-66. View abstract.
101. Homocysteine Lowering Trialists’ Collaboration. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomized trials. BMJ 1998;316:894-8. View abstract.
102. Van der Griend R, Biesma DH, Haas FJLM, et al. The effect of different treatment regimens in reducing fasting and postmethionine-load
103. Friso S, Jacques PF, Wilson PW, et al. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation 2001;103:2788-91. View abstract.
104. Lerner V, Miodownik C, Kaptsan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry 2001;158:1511-4. View abstract.
105. Bendich A, Cohen M. Vitamin B6 safety issues. Ann N Y Acad Sci 1990;585:321-30. View abstract.
106. Head KA. Peripheral neuropathy: Pathogenic mechanisms and alternative therapies. Altern Med Rev 2006;11:294-329. View abstract.
107. South M. Neonatal seizures after pyridoxine use — reply. Lancet 1999;354:2083.
108. Baxter P, Aicardi J. Neonatal seizures after pyridoxine use. Lancet 1999;354:2082-3. View abstract
109. Schaumburg H, Kaplan J, Windebank A. Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. N Engl J Med 1983;309:445-8. View abstract.
110. Gordon N. Pyridoxine dependency: an update. Dev Med Child Neurol 1997;39:63-5. View abstract.
111. Yates AA, Schlicker SA, Suitor CW. Dietary reference intakes: The new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J Am Diet Assoc 1998;98:699-706. View abstract.
Ashwagandha root extract
112. Bucci LR. Selected herbals and human exercise performance. Am J Clin Nutr 2000 72(suppl):624S–36S. paper
113. Singh N, Nath R, Lata A, et al. Withania somnifera (ashwagandha), a rejuvenating herbal drug which enhances survival during stress (an adaptogen). Int J Crude Drug Res 1982 20:29-35.
114. Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A review. Altern Med Rev 2000 5:334-346.
115. Provino R. The role of adaptogens in stress management. Australian Journal of Medical Herbalism 2010 22: 41-49.
116. Dhuley JN. Adaptogenic and cardioprotective action of Ashwagandha in rats and frogs. J Ethanopharmacol 2000 70: 57-63.
117. Raut AA, Rege NN, Tadvi FM, Solanki PV, Kene KR, Shirolkar SG, Pandey SN, Vaidya RA, Vaidya AB. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J Ayur Integrative Med 2012 3: 109-172.
118. Sandhu JS, Shah B, Shenoy S, Chauhan S, Lavekar GS, Padhi MM. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int J Ayurveda Res 2010 1:144-149.
119. Zovco Koncic M, Tomczyk, M. New Insights into Dietary Supplements Used in Sport: Active Substances, Pharmacological and Side Effects. Current Drug targets 2013 14:1079-1092.
120. Alam N, Monzur H, Khalil I, Moniruzzaman M, Sulaiman SA,Gan HS. Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Phytochem Res Published on line by Springer: DOI 10.1007/s11101-011-9221-5. 2011.http://link.springer.com/article/10.1007/s11101-011-9221-5#page-1
121. Archana R, Namasivayam A. Antistressor effect of Withania somnifera. J Ethnopharmacol 1999 64:91-3. View abstract. Paper and db 3711
122. Kulkarni SK, Dhir A. Withania somnifera: An Indian ginseng. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2008 32: 1093–1105.
123. Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med 2012 34:255-62.
124. Dongre S. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Improving Sexual Function in Women: a double?blind, randomized, placebo?controlled study. The electronic version of this article is at: http://www.harmonyforwomen.com/clinicals/ksm.pdf r
125. Ambiye VR, Langade D, Dongre S, Aptikar P, Kulkarni M, Dongre A. 2013. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania somnifera) in Oligospermic Males: A Pilot Study. Evidence-Based Complementary and Alternative Medicine Volume 2013, Article ID 571420, 6 pages. http://dx.doi.org/10.1155/2013/571420
126. Choudhary B, Shetty A, Langade DG. Efficacy of Ashwagandha (Withania somnifera [L.] Dunal) in improving cardiorespiratory endurance in healthy athletic adults. Ayu. 2015 36(1):63-8. doi: 10.4103/0974-8520.169002.
127. Chapman, PP, Whitehead JR, Binkkert RH. The 225-lb reps-to fatigue test as a submaximal estimate of 1-RM bench-press performance in college football players. J Strength Con Res 1998 12:258-261.
128. Brzycki M. Strength testing: predicting a one-rep max from repetitions to fatigue. JOPERD 1993 64:88-90.
129. Upton R, ed. Ashwagandha Root (Withania somnifera): Analytical, quality control, and therapeutic monograph. Santa Cruz, CA: American Herbal Pharmacopoeia 2000:1-25.
130. Bhattacharya SK, Satyan KS, Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J Exp Biol 1997;35: 236-9. View abstract.
131. Dasgupta A, Peterson A, Wells A, Actor JK. Effect of Indian Ayurvedic medicine Ashwagandha on measurement of serum digoxin and 11 commonly monitored drugs using immunoassays: study of protein binding and interaction with Digibind. Arch Pathol Lab Med 2007 131: 1298-303. View abstract.
132. Davis L, Kuttan G. Effect of Withania somnifera on cyclophosphamide-induced urotoxicity. Cancer Lett 2000 148:9-17. View abstract.
133. Davis L, Kuttan G. Suppressive effect of cyclophosphamide-induced toxicity by Withania somnifera extract in mice. J Ethnopharmacol 1998 62:209-14. View abstract
134. Cooley K, Szczurko O, Perri D, et al. Naturopathic care for anxiety: a randomized controlled trial ISRC TN78958974. PLoS One 2009 4:e6628. View abstract.
135. Sud Khyati S, Thaker B. A randomized double blind placebo controlled study of ashwagandha on generalized anxiety disorder. Int Ayurvedic Med J 2013 1(5):1-7.
136. Andallu B, Radhika B. 2000. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J Exp Biol 38:607-9.
137. Choudhary, D., Bhattacharyya, S., Joshi, K., “Body Weight Management in Adults Under Chronic Stress through Treatment with Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial”, Journal of Evidence-Based Complementary & Alternative Medicine 2106 22 96-106.
138. Choudhary, B., Shetty, A., Langade, D.G., “Efficacy of Ashwagandha (Withania somnifera (L.) Dunal) in improving cardiorespiratory endurance in healthy adults”, AYU, Journal of Research in Ayurveda 2015Vol 36 1: 63-68
139. Sachin, W., Langade, D., Joshi, K., Sinha, S.R., Bhattacharyya, S., “Examining the effects of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial”, Journal of the International Society of Sports Nutrition 2015 12:43, 11 pages
140. Cooley K, Szczurko O, Perri D, et al. Naturopathic care for anxiety: a randomized controlled trial ISRC TN78958974. PLoS One 2009;4:e6628. View abstract.
141. Agnihotri AP, Sontakke SD, Thawani VR, Saoji A, Goswami VS. Effects of Withania somnifera in patients of schizophrenia: a randomized, double blind, placebo controlled pilot trial study. Indian J Pharmacol. 2013 45(4):417-8. View abstract.
142. Chengappa KN, Bowie CR, Schlicht PJ, Fleet D, Brar JS, Jindal R. Randomized placebo-controlled adjunctive study of an extract of withania somnifera for cognitive dysfunction in bipolar disorder. J Clin Psychiatry. 2013 74(11):1076-83. View abstract.
143. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association’s Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997.
Cordyceps Extract
144. Robbers JE, Tyler VE. Tyler’s Herbs of Choice: The Therapeutic Use of Phytomedicinals. New York, NY: The Haworth Herbal Press, 1999.
145. Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I. J Altern Complement Med 1998;4:289-303. View abstract.
146. Zhu JS, Halpern GM, Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: part II. J Altern Complement Med 1998;4:429-57. View abstract.
147. Parcell AC, Smith JM, Schulthies SS, et al. Cordyceps sinensis (CordyMax Cs-4) supplementation does not improve endurance exercise performance. Int J Sport Nutr Exerc Metab 2004;14:236-42. View abstract
148. Chen, S., Li, Z., Krochmal, R., Abrazado, M., Kim, W., and Cooper, C. B. Effect of Cs-4 (Cordyceps sinensis) on exercise performance in healthy older subjects: a double-blind, placebo-controlled trial. J Altern.Complement Med 2010;16(5):585-590. View abstract.
149. Earnest, C. P., Morss, G. M., Wyatt, F., Jordan, A. N., Colson, S., Church, T. S., Fitzgerald, Y., Autrey, L., Jurca, R., and Lucia, A. Effects of a commercial herbal-based formula on exercise performance in cyclists. Med Sci Sports Exerc. 2004;36(3):504-509. View abstract.
150. Colson SN, Wyatt FB, Johnston DL, et al. Cordyceps sinensis- and Rhodiola rosea-based supplementation in male cyclists and its effect on muscle tissue oxygen saturation. J Strength Cond Res 2005;19:358-63. View abstract.
151. Yang WZ, Deng Xa Hu W. Treatment of sexual hypofunction with Cordyceps sinensis. Jiangxi Zhongyiyao. 1985;5:46-47.
152. Chen GZ, Chen GL, Sun T, et al. Effects of Cordyceps sinensis on murine T lymphocyte subsets. Chin Med J (English) 1991;104:4-8. View abstract.
153. Liu C, Lu S, Ji MR. [Effects of Cordyceps sinensis (CS) on in vitro natural killer cells]. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih 1992;12:267-9, 259. View abstract.
154. Xu RH, Peng XE, Chen GZ, Chen GL. Effects of cordyceps sinensis on natural killer activity and colony formation of B16 melanoma. Chin Med J (English) 1992;105:97-101. View abstract.
155. Chen YJ, Shiao MS, Lee SS, Wang SY. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sci 1997;60:2349-59. View abstract.
156. Zhu XY, Yu HY. [Immunosuppressive effect of cultured Cordyceps sinensis on cellular immune response]. Chung Hsi I Chieh Ho Tsa Chih 1990;10:485-7, 454. View abstract.
157. Chiu JH, Ju CH, Wu LH, et al. Cordyceps sinensis increases the expression of major histocompatibility complex class II antigens on human hepatoma cell line HA22T/VGH cells. Am J Chin Med 1998;26:159-70. View abstract.
158. Yamaguchi N, Yoshida J, Ren LJ, et al. Augmentation of various immune reactivities of tumor-bearing hosts with an extract of Cordyceps sinensis. Biotherapy 1990;2:199-205. View abstract.
159. Yoshida J, Takamura S, Yamaguchi N, et al. Antitumor activity of an extract of Cordyceps sinensis (Berk.) Sacc. against murine tumor cell lines. Jpn J Exp Med 1989;59:157-61. View abstract
160. Bok JW, Lermer L, Chilton J, et al. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999;51:891-8. View abstract.
161. Kuo YC, Tsai WJ, Shiao MS, et al. Cordyceps sinensis as an immunomodulatory agent. Am J Chin Med 1996;24:111-25. View abstract.
162. Kuo YC, Lin CY, Tsai WJ, et al. Growth inhibitors against tumor cells in Cordyceps sinensis other than cordycepin and polysaccharides. Cancer Invest 1994;12:611-5. View abstract.
163. Nakamura K, Yamaguchi Y, Kagota S, et al. Inhibitory effect of Cordyceps sinensis on spontaneous liver metastasis of Lewis lung carcinoma and B16 melanoma cells in syngeneic mice. Jpn J Pharmacol 1999;79:335-41. View abstract.
164. Li LS, Zheng F, Liu ZH. [Experimental study on effect of Cordyceps sinensis in ameliorating aminoglycoside induced nephrotoxicity]. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih 1996;16:733-7. View abstract.
165. Xu F, Huang JB, Jiang L, et al. Amelioration of cyclosporin nephrotoxicity by Cordyceps sinensis in kidney-transplanted recipients. Nephrol Dial Transplant 1995;10:142-3.
166. Bao ZD, Wu ZG, Zheng F. [Amelioration of aminoglycoside nephrotoxicity by Cordyceps sinensis in old patients]. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih 1994;14:271-3, 259. View abstract.
167. Cheng Q. [Effect of cordyceps sinensis on cellular immunity in rats with chronic renal insufficiency]. Chung Hua I Hsueh Tsa Chih (Taipei) 1992;72:27-9, 63. View abstract.
168. Zhao Y. [Inhibitory effects of alcoholic extract of Cordyceps sinensis on abdominal aortic thrombus formation in rabbits]. Chung Hua I Hsueh Tsa Chih (Taipei) 1991;71:612-5, 42. View abstract.
169. Mei QB, Tao JY, Gao SB, et al. [Antiarrhythmic effects of Cordyceps sinensis (Berk.) Sacc]. Chung Kuo Chung Yao Tsa Chih 1989;14:616-8, 640. View abstract.
170. Wang SM, Lee LJ, Lin WW, Chang CM. Effects of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplets in cultured rat adrenocortical cells. J Cell Biochem 1998;69:483-9. View abstract.
171. Kiho T, Yamane A, Hui J, et al. Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol Pharm Bull 1996;19:294-6. View abstract.
172. Kiho T, Hui J, Yamane A, Ukai S. Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol Pharm Bull 1993;16:1291-3. View abstract.
173. Chen JR, Yen JH, Lin CC, et al. The effects of Chinese herbs on improving survival and inhibiting anti-ds DNA antibody production in lupus mice. Am J Chin Med 1993;21:257-62. View abstract.
174. Zhou DH, Lin LZ. [Effect of Jinshuibao capsule on the immunological function of 36 patients with advanced cancer]. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih 1995;15:476-8. View abstract.
175. McGuffin M, Hobbs C, Upton R, Goldberg A, eds. American Herbal Products Association’s Botanical Safety Handbook. Boca Raton, FL: CRC Press, LLC 1997.
Eurycoma longfolia Extract (root) (LJ100)
176. Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia 2011 Jun 15. doi: 10.1111/j.1439-0272.2011.01168.x. [Epub ahead of print] . View abstract.
177. Tambi MI, Imran MK. Eurycoma longifolia jack in managing idiopathic male infertility. J Androl 2010;12:376-80. View abstract
178. Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia 2011 Jun 15. doi: 10.1111/j.1439-0272.2011.01168.x. [Epub ahead of print] . View abstract.
179. George, A., Henkel, R. “Phytoandrogenic properties of E. longifolia as natural alternative to testosterone replacement therapy”, Andrologia (2014) pp. 1-14
180. Ismail, S. B., Mohammad, W. M., Z. W., Geaorge, A., Hussain, N. H., N., Kamal, Z., M. M., Liske, E. “Randomized Clinical Trial on the Use of PHYSTA Freeze-Dried Water Extract of Eurycoma longifolia for the Improvement of Quality of Life and Sexual Well-Being in men”, Evidence-Based Complementary and Alternative Medicine (2012) Article ID 429268, 10 pages
181. Talbott, S., Talbott, J., Negrete, J., Jones, M., Roza, J., “Effect of Eurycoma longifolia Extract on Anabolic Balance During Endurance Exercise”, J of Intl Soc of Sport Med (2006), 1 page
182. Hamzah S and A Yusof. The ergogenic effects of Eurycoma longifolia Jack: a pilot study. British Journal of Sports Medicine 2003;37(5):465-466.
183. Muhamad AS, Chen CK, Ooi FK, and et al. Effects of Eurycoma longifolia Jack Supplementation on Recreational Athletes’ Endurance Running Capacity and Physiological Responses in the Heat. International Journal of Applied Sports Sciences 2010;22(1):119.
184. Jiwajinda S, Santisopasri V, Murakami A, et al. In vitro anti-tumor promoting and anti-parasitic activities of the quassinoids from Eurycoma longifolia, a medicinal plant in Southeast Asia. J Ethnopharmacol 2002;82:55-8. View abstract.
185. Bedir E, Abou-Gazar H, Ngwendson JN, Khan IA. Eurycomaoside: a new quassinoid-type glycoside from the roots of Eurycoma longifolia. Chem Pharm Bull (Tokyo) 2003;51:1301-3. View abstract.
186. Ang HH, Cheang HS. Effects of Eurycoma longifolia jack on laevator ani muscle in both uncastrated and testosterone-stimulated castrated intact male rats. Arch Pharm Res 2001;24:437-40. View abstract.
187. Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia 2011 Jun 15. doi: 10.1111/j.1439-0272.2011.01168.x. [Epub ahead of print] . View abstract.
188. Zanoli P, Zavatti M, Montanari C, Baraldi M. Influcence of Eurycoma longifolia on the copulatory activity of sexually sluggish and impotenet male rats. J Ethnopharmacol 2009;126:308-13. View abstract.
189. Ang HH, Sim MK. Eurycoma longifolia Jack enhances libido in sexually experienced male rats. Exp Anim 1997;46:287-90. View abstract.
190. Ang HH, Ngai TH. Aphrodisiac evaluation in non-copulator male rats after chronic administration of Eurycoma longifolia Jack. Fundam Clin Pharmacol 2001;15:265-8. View abstract.
191. Ang HH, Ikeda S, Gan EK. Evaluation of the potency activity of aphrodisiac in Eurycoma longifolia Jack. Phytother Res 2001;15:435-6. View abstract.
192. Ang HH, Cheang HS, Yusof AP. Effects of Eurycoma longifolia Jack (Tongkat Ali) on the initiation of sexual performance of inexperienced castrated male rats. Exp Anim 2000;49:35-8. View abstract.
193. Ang HH, Lee KL. Effect of Eurycoma longifolia Jack on orientation activities in middle-aged male rats. Fundam Clin Pharmacol 2002;16:479-83. View abstract.
194. Bhat R, Karmin AA. Tongkat ali (Eurycoma longifolia jack): a review on its ethnobotany and pharmacological importance. Fitoterapia 2010;81:669-79. View abstract.
195. Wahab NA, Mokhtar NM, Halim WN, Das S. The effect of Eurycoma longifolia jack on spermatogenesis in estrogen-treated rats. Clinics 2010;65:93-8. View abstract.
196. Qinna N, Taha H, Matalka KZ, Badwan AA. A new herbal combination, Etana, for enhancing erectile function: an efficacy and safety study in animals. International Journal of Impotence Research 2009;21:315-20. View abstract.
197. Ang HH, Cheang HS. Studies on the anxiolytic activity of Eurycoma longifolia Jack roots in mice. Jpn J Pharmacol 1999;79:497-500. View abstract.
Pine Sterols (Beta-Sitosterols)
198. Anon. FDA authorizes new coronary heart disease health claim for plant sterol and plant stanol esters. FDA. 2000. Available at:http://www3.scienceblog.com/community/older/archives/M/1/fda0642.htm.
199. Berges RR, Windeler J, Trampisch HJ, et al. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet 1995;345:1529-32. View abstract.
200. Klippel KF, Hiltl DM, Schipp B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. Br J Urol 1997;80:427-32. View abstract.
201. Wilt TJ, MacDonald R, Ishani A. beta-sitosterol for the treatment of benign prostatic hyperplasia: a systematic review. BJU Int 1999;83:976-83. View abstract.
202. Lichtenstein AH, Deckelbaum RJ. Stanol/sterol ester-containing foods and blood cholesterol levels: a statement for healthcare professionals from Nutrition Committee, Council on Nutrition, Physical Activity, Metabolism of American Heart Association. Circulation 2001;103:1177-9. View abstract.
203. Berges RR, Kassen A, Senge T. Treatment of symptomatic benign prostatic hyperplasia with beta-sitosterol: an 18-month follow-up. BJU Int 2000;85:842-6. View abstract.
204. Wilt, T., Ishani, A., MacDonald, R., Stark, G., Mulrow, C., and Lau, J. Beta-sitosterols for benign prostatic hyperplasia. Cochrane.Database.Syst.Rev 2000;(2):CD001043. View abstract.
205. Kadow, C. and Abrams, P. H. A double-blind trial of the effect of beta-sitosteryl glucoside (WA184) in the treatment of benign prostatic hyperplasia. Eur.Urol. 1986;12(3):187-189. View abstract.
206. Puato, M., Faggin, E., Rattazzi, M., Zambon, A., Cipollone, F., Grego, F., Ganassin, L., Plebani, M., Mezzetti, A., and Pauletto, P. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin-based regimen in patients undergoing carotid endarterectomy. Stroke 2010;41(6):1163-1168. View abstract.
207. Matvienko OA, Lewis DS, Swanson M, et al. A single daily dose of soybean phytosterols in ground beef decreases serum total cholesterol and LDL cholesterol in young, mildly hypercholesterolemic men. Am J Clin Nutr 2002;76:57-64. View abstract.
208. Law M. Plant sterol and stanol margarines and health. BMJ 2000;320:861-4. View abstract.
209. Cabeza M, Bratoeff E, Heuze I, et al. Effect of beta-sitosterol as inhibitor of 5 alpha-reductase in hamster prostate. Proc West Pharmacol Soc 2003;46:153-5.
210. Kassen A, Berges R, Senge T, et al. Effect of beta-sitosterol on transforming growth factor-beta-1 expression and translocation protein kinase C alpha in human prostate stromal cells in vitro. Eur Urol 2000;37:735-41. . View abstract.
211. Becker M, Staab D, Von Bergmann K. Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. J Pediatr 1993;122:292-6. View abstract.
212. Oster P, Schlierf G, Heuck CC, et al. [Sitosterol in familial hyperlipoproteinemia type II. A randomized, double-blind, cross-over study]. Dtsch Med Wochenschr 1976;101:1308-11. View abstract.
213. Schlierf G, Oster P, Heuck CC, et al. Sitosterol in juvenile type II hyperlipoproteinemia. Atherosclerosis 1978;30:245-8. View abstract.
214. Schwartzkopff W, Jantke HJ. [Dose-effect of beta-sitosterin in type IIa and IIb hypercholesterolemias]. MMW Munch Med Wochenschr 1978;120:1575-8. View abstract.
215. Becker M, Staab D, Von Bergman K. Long-term treatment of severe familial hypercholesterolemia in children: effect of sitosterol and bezafibrate. Pediatrics 1992;89:138-42. View abstract.
216. Weststrate JA, Meijer GW. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 1998;52:334-43. View abstract.
217. Donald PR, Lamprecht JH, Freestone M, et al. A randomised placebo-controlled trial of the efficacy of beta-sitosterol and its glucoside as adjuvants in the treatment of pulmonary tuberculosis. Int J Tubercul Lung Dis 1997;1:518-22. View abstract.
218. Gerolami A, Sarles H. Letter: Beta-sitosterol and chenodeoxycholic acid in the treatment of cholesterol gallstones. Lancet 1975;2:721.
219. Tangedahl TN, Thistle JL, Hofmann AF, et al. Effect of beta-sitosterol alone or in combination with chenic acid on cholesterol saturation of bile and cholesterol absorption in gallstone patients. Gastroenterol 1979;76:1341-6.
220. Neil HA, Meijer GW, Roe LS. Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterol-enriched fat spread. Atherosclerosis 2001;156:329-37. View abstract.
Agmatine Sulphate
221. Examine.com Dietary Supplement Guide. https://examine.com/
222. Keynan O, et al. Safety and Efficacy of Dietary Agmatine Sulfate in Lumbar Disc-associated Radiculopathy. An Open-label, Dose-escalating Study Followed by a Randomized, Double-blind, Placebo-controlled Trial. Pain Med 2010; 11(3):356-68. doi: 10.1111/j.1526-4637.2010.00808.x.
223. Shopsin, B. (2013). The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: A pilot study. Acta Neuropsychiatrica 25(2), 113-118. doi:10.1111/j.1601-5215.2012.00675.x
224. Krass M, et al. Antidepressant-like effect of agmatine is not mediated by serotonin. Behav Brain Res. 2008; 188(2):324-8. doi: 10.1016/j.bbr.2007.11.013. Epub 2007 Nov 24.
225. Naila A, et al. Control of biogenic amines in food–existing and emerging approaches. J Food Sci. 2010 75(7):R139-50. doi: 10.1111/j.1750-3841.2010.01774.x.
226. Molderings GJ, Haenisch B. Agmatine (decarboxylated L-arginine): physiological role and therapeutic potential. Pharmacol Ther. 2012 133(3):351-65. doi: 10.1016/j.pharmthera.2011.12.005. Epub 2011 Dec 22.
227. Holt A, Baker GB. Metabolism of agmatine (clonidine-displacing substance) by diamine oxidase and the possible implications for studies of imidazoline receptors.Prog Brain Res. 1995 106:187-97
228. Molderings GJ, et al. Exposure of rat isolated stomach and rats in vivo to {(14)C}agmatine: accumulation in the stomach wall and distribution in various tissues.Fundam Clin Pharmacol. 2002 16(3):219-25.
229. Li YF, et al. Antidepressant-like effect of agmatine and its possible mechanism. Eur J Pharmacol. 2003 469(1-3):81-8.
230. Roberts JC, et al. Pharmacodynamic and pharmacokinetic studies of agmatine after spinal administration in the mouse. J Pharmacol Exp Ther. 2005 314(3):1226-33. Epub 2005 Jun 2.
231. Raasch W, et al. Agmatine, an endogenous ligand at imidazoline binding sites, does not antagonize the clonidine-mediated blood pressure reaction. Br J Pharmacol. 2002 135(3):663-72.
232. Reis DJ, Regunathan S. Agmatine: a novel neurotransmitter. Adv Pharmacol. 1998 42:645-9.
233. Sastre M, et al. Agmatinase activity in rat brain: a metabolic pathway for the degradation of agmatine. J Neurochem. 1996 67(4):1761-5.
234. Otake K, et al. Regional localization of agmatine in the rat brain: an immunocytochemical study. Brain Res. 1998 16;787(1):1-14
235. Goracke-Postle CJ, et al. Release of tritiated agmatine from spinal synaptosomes. Neuroreport. 2006 23;17(1):13-7.
236. Sastre M, Regunathan S, Reis DJ. Uptake of agmatine into rat brain synaptosomes: possible role of cation channels. J Neurochem. 1997 69(6):2421-6.
237. Goracke-Postle CJ, et al. Potassium- and capsaicin-induced release of agmatine from spinal nerve terminals. J Neurochem. 2007 102(6):1738-48. Epub 2007 Jun 1.
238. Goracke-Postle CJ, et al. Agmatine transport into spinal nerve terminals is modulated by polyamine analogs. J Neurochem. 2007 100(1):132-41.
239. Regunathan S, et al. Agmatine (decarboxylated arginine) is synthesized and stored in astrocytes. Neuroreport. 1995 100(1):132-41.
240. Zomkowski AD, Santos AR, Rodrigues AL. Putrescine produces antidepressant-like effects in the forced swimming test and in the tail suspension test in mice.Prog Neuropsychopharmacol Biol Psychiatry. 2006 2006 Dec 30;30(8):1419-25. Epub 2006 Jul 5.
241. Molderings GJ, et al. Dual interaction of agmatine with the rat alpha(2D)-adrenoceptor: competitive antagonism and allosteric activation. Br J Pharmacol. 2000 130(7):1706-12.
242. Dias Elpo Zomkowski A, et al. Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test.Brain Res. 2004 1023(2):253-63.
243. Gibson DA, et al. Radioligand binding studies reveal agmatine is a more selective antagonist for a polyamine-site on the NMDA receptor than arcaine or ifenprodil. Brain Res. 2002 11;952(1):71-7.
244. Lewin AH, et al. Molecular features associated with polyamine modulation of NMDA receptors. J Med Chem. 1998 12;41(6):988-95.
245. Jiang XZ, et al. 5-HT1A/1B receptors, alpha2-adrenoceptors and the post-receptor adenylate cyclase activation in the mice brain are involved in the antidepressant-like action of agmatine. Yao Xue Xue Bao. 2008 43(5):467-73.
246. Demady DR, et al. Agmatine enhances the NADPH oxidase activity of neuronal NO synthase and leads to oxidative inactivation of the enzyme. Mol Pharmacol. 2001 59(1):24-9.
247. Nishida CR, Ortiz de Montellano PR. Electron transfer and catalytic activity of nitric oxide synthases. Chimeric constructs of the neuronal, inducible, and endothelial isoforms. J Biol Chem. 1998 6;273(10):5566-71.
248. Raghavan, S. A. V., Dikshit, M. “Vascular regulation by the l-arginine metabolites, nitric oxide and agmatine” Pharmacological Research (2004) 49: pp 397–414
249. Morrissey JJ, Klahr S. Agmatine activation of nitric oxide synthase in endothelial cells. Proc Assoc Am Physicians. 1997 109(1):51-7.
250. Haulic? I, et al. Preliminary research on possible relationship of NO with agmatine at the vascular level. Rom J Physiol. 1999 36(1-2):67-79.
251. Joshi MS, et al. Receptor-mediated activation of nitric oxide synthesis by arginine in endothelial cells. Proc Natl Acad Sci U S A. 2007 104(24):9982-7. Epub 2007 May 29.
252. Piletz JE, Chikkala DN, Ernsberger P. Comparison of the properties of agmatine and endogenous clonidine-displacing substance at imidazoline and alpha-2 adrenergic receptors. J Pharmacol Exp Ther. 1995 272(2):581-7.
253. Chang CH, et al. Increase of beta-endorphin secretion by agmatine is induced by activation of imidazoline I(2A) receptors in adrenal gland of rats. Neurosci Lett. (2010)
254. Zhu MY, et al. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell Mol Neurobiol. 2003 14;468(3):297-9. doi: 10.1016/j.neulet.2009.11.018. Epub 2009 Nov 12.
255. Wade CL, et al. Immunoneutralization of agmatine sensitizes mice to micro-opioid receptor tolerance. J Pharmacol Exp Ther. 2009 331(2):539-46. doi: 10.1124/jpet.109.155424. Epub 2009 Aug 14.
256. Burban A, et al. Histamine potentiates N-methyl-D-aspartate receptors by interacting with an allosteric site distinct from the polyamine binding site. J Pharmacol Exp Ther. 2010 332(3):912-21. doi: 10.1124/jpet.109.158543. Epub 2009 Dec 15.
257. Wang CC, et al. Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol. 2010 6;10:11. doi: 10.1186/1471-2210-10-11.
258. Chin HM, et al. Agmatine reduced the expressions of nitric oxide synthase and peroxynitrite formation in rat cerebral cortex after transient global cerebral ischemia. 43(3): 230–240. doi: 10.5115/acb.2010.43.3.230.
259. Kim JH, et al. Agmatine attenuates brain edema through reducing the expression of aquaporin-1 after cerebral ischemia. J Cereb Blood Flow Metab. 2010 30(5):943-9. doi: 10.1038/jcbfm.2009.260. Epub 2009 Dec 23.
260. Lee WT, et al. Neuroprotective effects of agmatine on oxygen-glucose deprived primary-cultured astrocytes and nuclear translocation of nuclear factor-kappa B.Brain Res. 2009 24;1281:64-70. doi: 10.1016/j.brainres.2009.05.046. Epub 2009 May 22.
261. Kim JH, et al. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004 189(1):122-30.
262. Feng Y, Piletz JE, Leblanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2002 ;52(4):606-11.
263. Reis DJ, Yang XC, Milner TA. Agmatine containing axon terminals in rat hippocampus form synapses on pyramidal cells. Neurosci Lett. 1998 10;250(3):185-8.
264. Seo S, Liu P, Leitch B. Spatial learning-induced accumulation of agmatine and glutamate at hippocampal CA1 synaptic terminals. Neuroscience. 2011 29;192:28-36. doi: 10.1016/j.neuroscience.2011.07.007. Epub 2011 Jul 18.
265. Feng Y, Halaris AE, Piletz JE. Determination of agmatine in brain and plasma using high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1997 11;691(2):277-86.
266. Betancourt L, et al. In vivo monitoring of cerebral agmatine by microdialysis and capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. (2012)
267. Liu P, et al. Spatial learning results in elevated agmatine levels in the rat brain. Hippocampus. 2008 1;880(1):58-65. doi: 10.1016/j.jchromb.2011.11.016. Epub 2011 Nov 18.
268. Leitch B, et al. Spatial learning-induced increase in agmatine levels at hippocampal CA1 synapses. Synapse. 2011 1;880(1):58-65. doi: 10.1016/j.jchromb.2011.11.016. Epub 2011 Nov 18.
269. Rushaidhi M, et al. Participation of hippocampal agmatine in spatial learning: an in vivo microdialysis study. Neuropharmacology. 2013 65:200-5. doi: 10.1016/j.neuropharm.2012.10.007. Epub 2012 Oct 29.
270. Wang ZM, et al. Effects of agmatine on neuronal discharges in rat hippocampal CA1 area. Sheng Li Xue Bao. 2003 25;55(6):717-21.
Maca Extract
271. National Academy of Science. Lost Crops of the Incas Little-Known Plants of the Andes with Promise for Worldwide Cultivation (1989). Available at: http://books.nap.edu/books/030904264X/html/57.html
272. Ganzera M, Zhao J, Muhammad I, Khan IA. Chemical profiling and standardization of Lepidium meyenii (Maca) by reversed phase high performance liquid chromatography. Chem Pharm Bull (Tokyo) 2002;50:988-99.. View abstract.
273. Gonzales GF, Cordova A, Gonzales C, et al. Lepidium meyenii (Maca) improved semen parameters in adult men. Asian J Androl 2001;3:301-3. View abstract.
274. Gonzales GF, Cordova A, Vega K, et al. Effect of Lepidium meyenii (MACA) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men. Andrologia 2002;34:367-72.. View abstract.
275. Stone, M., Ibarra, A., Roller, M., Zangara, A., Stevenson, E. “A pilot investigation into the effects of maca supplementation on physical activity and sexual desire in sportsmen”, Journal of Ethnopharmacology 126 (2009) pp. 574-576
276. Shin, B-C., Lee, M. S., Yang, E. J., Lim, H-S., Ernst, E. “Maca (L. meyenii) for improving sexual function: a systemic review”, BMC Complementary & Alternative Medicine (2010) 10:44 6 pages
277. Li G, Ammermann U, Quiros CF. Gluconsinolate contents in Maca (Lepidium peruvianum Chacon) seeds, sprouts, mature plants, and several derived commercial products. Economic Botany 2001;55:255-62
278. Gonzales GF, Cordova A, Vega K, et al. Effect of Lepidium meyenii (Maca), a root with aphrodisiac and fertility-enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol 2003;176:163-168.. View abstract.
279. Zheng BL, He K, Kim CH, et al. Effect of a lipidic extract from lepidium meyenii on sexual behavior in mice and rats. Urology 2000;55:598-602.
280. Piacente S, Carbone V, Plaza A, et al. Investigation of the tuber constituents of maca (Lepidium meyenii Walp.). J Agric Food Chem 2002;50:5621-25.. View abstract.
281. Dording CM, Schettler PJ, Dalton ED, Parkin SR, Walker RS, Fehling KB, Fava M,Mischoulon D. A double-blind placebo-controlled trial of maca root as treatment for antidepressant-induced sexual dysfunction in women. Evid Based Complement Alternat Med 2015;2015:949036. View abstract.
American Ginseng Extract
282. Hsu CC, Ho MC, Lin LC, et al. American ginseng supplementation attenuates creatine kinase level induced by submaximal exercise in human beings. World J Gastroenterol 2005;11:5327-31. View abstract.
283. Morris AC, Jacobs I, McLellan TM, et al. No ergogenic effect of ginseng ingestion. Int J Sport Nutr 1996;6:263-71. View abstract.
284. Scholey A, Ossoukhova A, Owen L, et al. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology (Berl) 2010;212(3):345-56. View abstract.
285. Lim W, Mudge KW, Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium). J Agric Food Chem 2005;53:8498-505. View abstract.
286. Wang X, Sakuma T, Asafu-Adjaye E, Shiu GK. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal Chem 1999;71:1579-84.. View abstract.
287. Sievenpiper JL, Arnason JT, Leiter LA, Vuksan V. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: the role of ginsenosides. J Am Coll Nutr 2004;23:248-58. View abstract.
288. Sengupta S, Toh SA, Sellers LA,
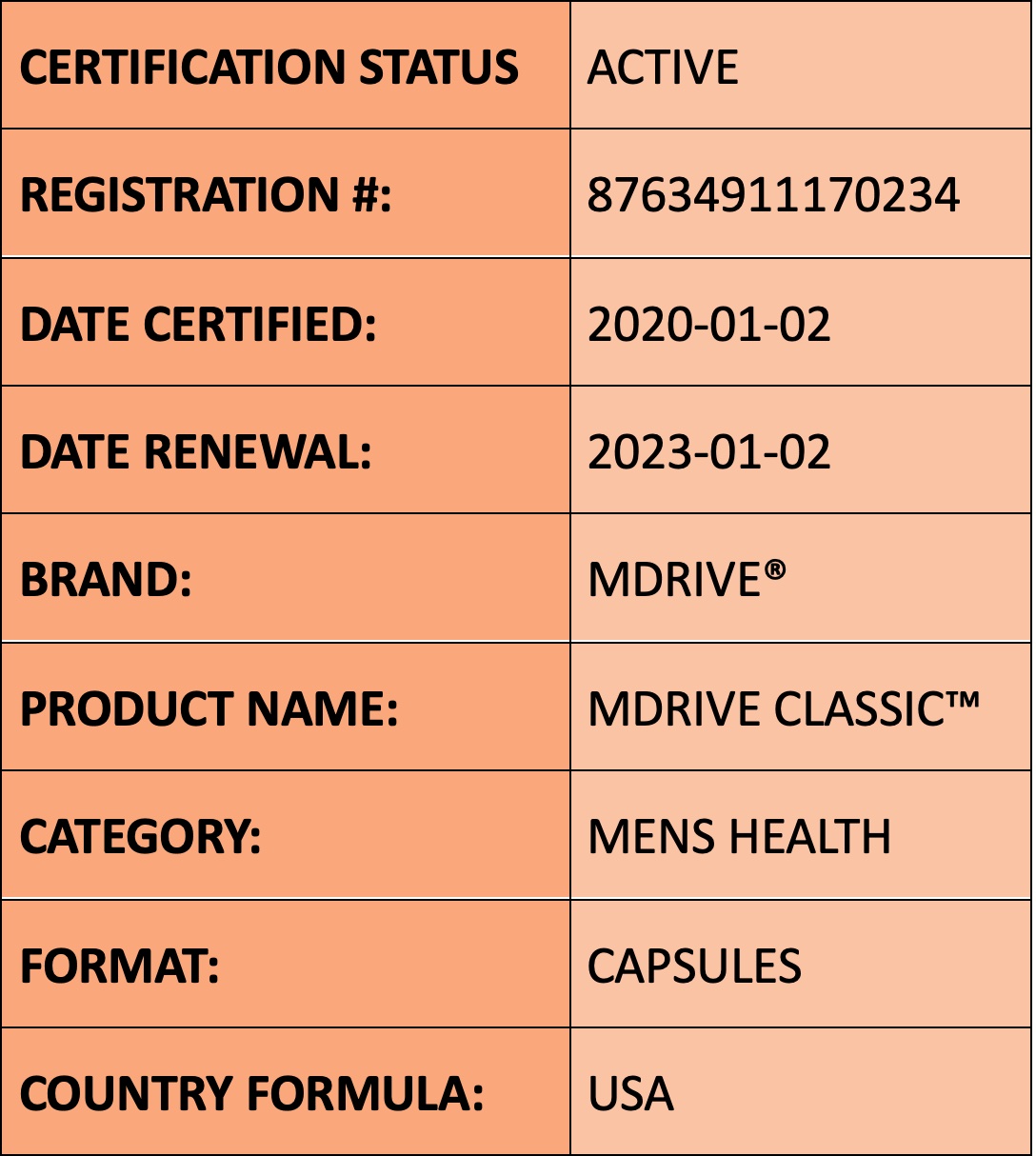
STRONGSCIENCE® is a third-party certification mark certifying that this product’s claims, dosing, and safety have been scientifically reviewed and are science-backed. Scanning the QR next to the certification mark brings you to this product page. Showing you the certification mark level and the claims or dosage that have been scientifically reviewed. In addition, see the status of the certification, certified date, renewal date, product-specific registration # and more. Easily accessible are the product overview, ingredients, and most importantly Research Transparency™. Here you shall find the references used when researching the science behind the product that was certified. We provide additional product information so you can make a better buying decision. Click the Buy Now link to order online.
Scottsdale, Arizona
Las Vegas, Nevada